Inside the COVID-19 Testing Advancements That Could Be Key to College Sports
Joel Erdmann is not that kind of Ph.D. The South Alabama athletic director holds a doctorate in sports management—not molecular science.
But even he understands the importance of the latest advancements in COVID-19 testing. In fact, he’s anxiously awaiting the arrival of a toaster-sized testing machine that will produce results within 15 minutes and cost half the rate of a standard PCR test.
He’s not alone. Roughly half the athletic directors in the Sun Belt are waiting on the same. Conference schools say they spent a combined $500,000 to $1 million on testing kits to pair with the six-pound Abbott ID NOW machines. The test kits and machines should arrive this week, giving administrators “a quick test at our fingertips,” says Erdmann.
As Week 1 in college football arrives, so do thousands of rapid, point-of-care COVID-19 tests for the dozens of programs planning to play this year. While these tests aren’t significantly impactful to a fall football season—the ID Now machine, for instance, can process only one test at a time—the true game-changers aren’t far behind, experts say. Quickly moving down the pipeline is a wave of new-generation rapid tests set up for at-site results (point of care). One doctor is even hopeful that a self-administering at-home test—“the Holy Grail,” he calls it—could be available by the time March Madness is scheduled to start.
But that’s not even the best news. Abbott has a newer innovation that has college administrators and doctors alike buzzing. The Abbott BinaxNOW is even quicker, cheaper and potentially more accurate than the ID NOW. And though it’s not yet widely available—and might not be until late this fall—industry experts believe the BinaxNOW is a true game-changer for society and sports. Even the most cautious of physicians are excited about the possibilities of a device they compare to a drug store pregnancy test. “It seems like this is something that can really help,” says Doug Aukerman, Oregon State’s team doctor and the head of the Pac-12’s medical advisory panel. “It does not require a machine to read it. It does not require a lab. This is a cartridge which has a color change in the strip.”
The developments in the testing industry are significant for the completion of a 2020–21 college sports year, and Abbott’s latest innovation could potentially help a fall football season reach the finish line—or, for some, get a season started. On Tuesday, President Donald Trump and commissioner Kevin Warren held a phone call about how the league could return to playing football as soon as possible. At the heart of their conversation, according to reports, was the White House’s intent to distribute Abbott’s newest rapid tests to Big Ten schools.
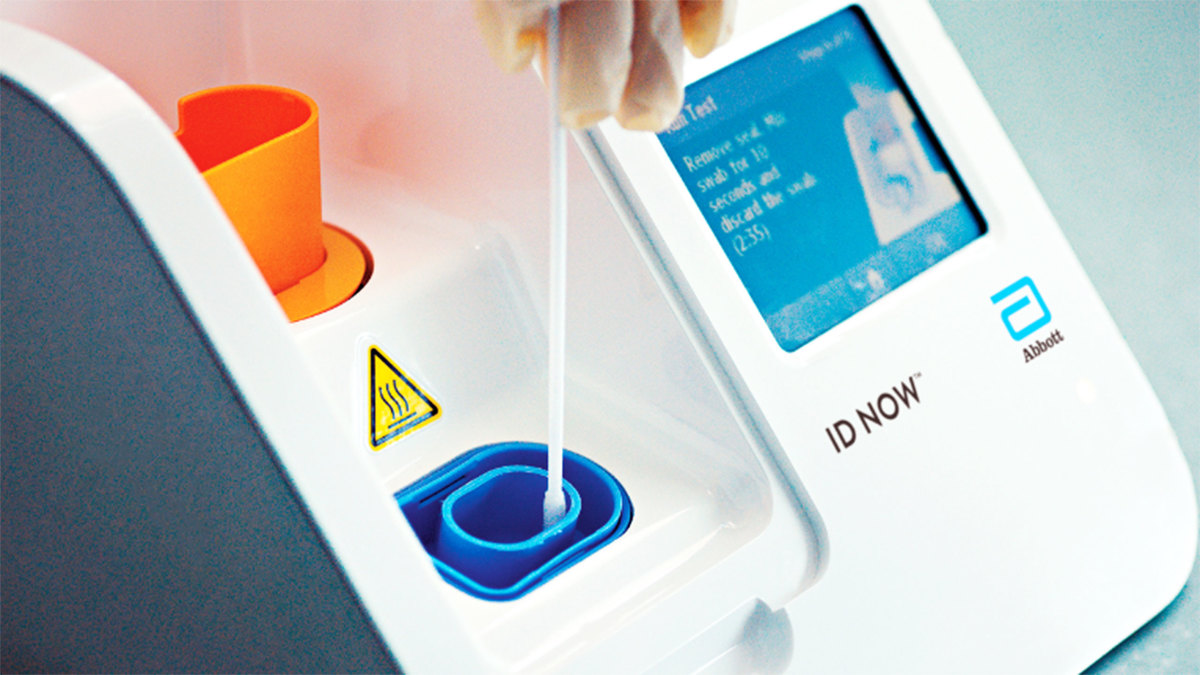
While Abbott’s BinaxNOW is all the rage, the ID NOW is on its way to benefiting several college athletic departments immediately. At least half of the Sun Belt’s 10 members were part of a bulk order of the ID NOW machines and testing kits made through the conference office. The ID NOW machines came at a steep discount with a requirement that schools purchase at least 2,000 tests at roughly $65 a pop—a total of $130,000. Some schools bought as many as 3,000 tests and are expected to receive two machines.
A machine can process one test in about 15 minutes or four to five in an hour. Two machines can process eight to 10 in an hour. That’s a low number for football rosters with more than 100 players and at least 20 staff members. Several schools will use BinaxNOW to supplement the more expensive and time-consuming lab-based PCR tests, but at least one athletic department plans to use ID NOW as its primary testing device. Georgia Southern will test players and coaches over a three-day window, starting Wednesday of game week and running through Friday.
One of the ID NOW’s biggest benefits is the ability to bypass a lab process that can take 24–72 hours to produce results. That’s how it significantly differs from another advancement approved by the FDA a couple of weeks ago, SalivaDirect, which garnered hype that was overblown, physicians say. While SalivaDirect makes collection easier—specimens are acquired by spitting into a vial—it still must be processed at a lab, opening up the possibility for dreaded delays in result return time. “I don’t think I’ve seen a larger media blunder and hype blunder than the SalivaDirect test,” says Michael Mina, assistant professor of epidemiology at Harvard. “There is no magic rapid test underlining SalivaDirect.”
The Abbott ID NOW not only produces rapid results but is also considered a PCR test—only marked down in cost and with a quicker turnaround than the standard, nasopharyngeal PCR examine. While a nurse is needed to administer the test—a nasal swab that is not as intrusive as the standard PCR—ID NOW can be processed quickly in student health laboratories on campus. “The challenge is sending off your tests to a lab and then if you’re traveling [for a game], you’re waiting on the results before you leave campus,” says GSU athletic director Jared Benko. “With this, you don’t have that wait.”
ID NOW is maybe better suited as a weekly surveillance test for sports with smaller rosters, such as basketball and volleyball. Also, the ID NOW is mobile enough for road trips and affords administrators the opportunity to quickly test any symptomatic individuals on game day. “This is going to help us in unique situations,” says Erdmann, whose South Alabama program kicks off the season at Southern Miss on Thursday night in the first 2020 game pitting two FBS programs against each other.
For all the positive vibes of the Abbott ID NOW, the test sacrifices accuracy for speed and cost. The accuracy ranges greatly depending on the study you choose to read—some in the 80th percentile and others are as high as 98%. “It’s about as sensitive as the best antigen,” says Ben Pinsky, the medical director of clinical virology laboratory at Stanford and an expert in the COVID-19 testing industry.
While PCR tests identify the virus genome, antigen tests search for virus proteins. The latter is considered less ideal, but faster in turnaround time and often cheaper. The ID NOW is somewhat of a combination of the two. Most college football conferences plan to use both tests as part of a thrice-weekly in-season testing protocol—two PCR tests on Monday and Thursday, for example, and one rapid antigen test within 24 hours of a game. One of the most popular antigen options is the Quidel Sofia, which can process 40 tests within an hour. While its accuracy is in the mid-90s, physicians say antigen tests can be misleading. “People are pretty much just accepting the warts,” says Geoffrey Baird, interim chair of the department of laboratory medicine and pathology at the University of Washington.
Unlike the five other conferences planning to play, the Sun Belt does not plan to require its teams to test more than the NCAA standard once a week—a decision rooted in both available school resources and testing supplies, says Jeffrey Dugas, Troy’s team doctor and an orthopedic surgeon in Birmingham who chairs the Sun Belt’s COVID-19 advisory panel. He’s the person who connected the league to a third-party vendor for the discounted ID NOW machines. “There are obviously resource differences between the SEC and Sun Belt,” he says. “To do three tests a week for us doesn’t make sense. I don’t think we can mandate that, just resource-wise. The optics are good, but I don’t think it changes anything for you in terms of what you can and can’t do.”
Meanwhile, Abbott’s newest innovation, BinaxNOW, could possibly meet what infectious disease experts describe as the trifecta in testing: speed, cost and accuracy. Priced at $5 a test, BinaxNOW needs no machinery, using instead a handheld portable tool. A nasal swab specimen is applied to the credit-card sized tool that, a few minutes later, produces a result via a color strip.
According to Abbott, the test demonstrated an accuracy rate of 97%–99%, an exceptional rate compared with other point-of-care antigen tests, which can range from 70%–95% accuracy, physicians say. “Think about a team going on a road trip. You can take a test with you and do the test the day of the game without having to lug a giant machine,” Aukerman says. “We talked many months ago about what we need to be looking for to tell if football is going to happen. My comment was accurate point-of-care testing.”
It’s here. Well, sort of.
Acquiring BinaxNOW isn’t so quick and easy. While administrators expect them to arrive on their campuses potentially toward the end of the fall football season, others aren’t so optimistic. The government procured at least one-third of the BinaxNOW testing kits, says Dugas, and he estimates that they may not be readily available to athletic departments until spring.

However, while it might be the first of its kind, BinaxNOW won’t be the last, says Thomas Huard, chief clinical laboratory advisor at Campus Health Project. The organization has entered into contracts with two rapid point-of-care COVID-19 tests, both of which are in the exploratory stages and have not been granted FDA approval. Huard believes they will significantly boost the chances for a fall college football season. Because of nondisclosure agreements, he declined to reveal specifics, giving only general details of the two testing advancements—both of which can process multiple samples at once and produce results within an hour at a cost similar to a standard lab-based PCR test—roughly $100 a pop.
One can even process 96 samples in as little time as 30 minutes using a machine that’s roughly the size of a laptop. “That’s going to be a game-changer,” says Huard, whose CHP company is overseeing testing for several colleges, including the University of Texas. “I’m optimistic that both are going to be available in the next few weeks. They will be very competitive. As soon as they are released, they will be all acquired, and then you’ll have to manufacture more.”
That’s where things get tricky. The availability of point-of-care testing kits and PCR testing supplies is an issue. Even the Abbott ID NOW isn’t necessarily readily available, despite the Sun Belt’s haul. Huard calls them “difficult to obtain” and says the U.S. Department of Defense has acquired “as many as they can get their hands on.”
The federal government ships a large portion of testing resources to hotspot cities, hospitals and nursing homes, physicians say. Shortages of testing resources are commonplace in labs across the country, especially the supplies connected to the more intrusive PCR tests. Most of those resources—nasal swabs, plastic consumables, liquid reagents—are single-use.
The infamous delays in return times are rooted in insufficient supply and labs that have reached their testing capacity. This creates backlogs, turning a 24-hour return time to 72 hours. Physicians don’t expect lab issues to improve this fall as the country enters the flu season while juggling a pandemic. Lab administrators in Texas, California, Utah and Washington told Sports Illustrated they expect their testing capacity to increase significantly this fall. Huard anticipates a “big strain” on labs that will have an impact on athletic programs. “It’s like you might want to ask, ‘Why can’t they make a billion of these?!’ Well, they are, and we’re all using them,” says Baird.
For this reason, innovations and advancements in accurate point-of-care tests are essential. You bypass the lab. In the meantime, research groups are working on at-home, self-administered tests. Pinsky believes those could be available in six months. “That’s optimistic,” he says, “but it’s O.K. to be optimistic.”
All of this said, testing is not foolproof and it doesn’t come without ethical dilemmas. Many doctors are asking themselves why thousands of healthy, asymptomatic adults are using testing resources that could be used for those in need. “There are not enough tests capability right now to test all the people we want with the frequency we want,” says Baird. “You have to ask yourselves, ‘Is that ethical—paying for healthy athletes to get to the front of the line over sick people?’ That’s not a great look.”
Sankar Swaminathan, chief of the infectious diseases department at the Utah School of Medicine, is a member of the Pac-12’s COVID-19 advisory panel and was involved in the league’s discussion to cancel a fall season. He sees flaws in the in-season weekly testing protocols for the six FBS conferences still attempting to play. It normally takes three to five days after contracting COVID-19 for a person to begin shedding the virus and registering a positive result.
Even in a thrice-weekly testing plan, there are windows for the possibility of a positive to go undetected before teams collide with another team on the field. “Therein lies the problem,” says Swaminathan. “You need an accurate point-of-care test right before the game.”
The testing industry continues moving closer to achieving that milestone. Until then, Swaminathan has a message for those teams planning to play this fall.
“Good luck,” he says. “I wouldn’t want to predict anything. I hope they succeed and nobody gets sick. But I wouldn’t bet on it.”
More College Football Coverage From SI.com
Has Any Team Had More Rapid Turnover Than LSU in 2020?
Ranking the Best Alabama Players of the Nick Saban Era
Top Ohio State Football Recruits in Program History
